
Somatropin or the growth hormone (GH) is one of the most abundant hormones produced by the anterior pituitary lobe. It is synthesized and secreted by specific cells, the somatotropes. GH daily secretion varies through life. Its concentration is high in children, reaching maximum levels in adolescence, while in adulthood, the concentration decreases progressively. GH secretion occurs in pulsatile and irregular way; between pulses, the flowing GH decreases up to levels that are sometimes non-detectable through some of the current methodologies. Secretion pulse amplitude achieves maximum levels at night and the most constant GH secretion occurs little after deep sleep onset.
PDgrowth® contains somatropin, the recombinant human growth hormone, whose amino acid sequence is identical to the natural growth hormone. PDgrowth® active ingredient is made up of a unique 191 amino acid polypeptide chain. Its molecular weight is 22,124 Da. It contains two disulphide bonds and it is not glycosylated. It is produces by recombinant DNA technology.
– Lyophilized vial: 4IU
– Solvent ampule: 1ml Distilled water for injection
– Lyophilized powder: Sterile and white
– Solvent: Colorless – Clear – Without particle
– Effect on Ability to Drive and Operate Machinery
Somatropin-containing products have no influence on the ability to drive and use machines.
– Overdosage
If you use too much PDgrowth® you may need medical treatment right away. Too much PDgrowth® can cause initial hypoglycemia, followed by hyperglycemia.
– If you forget to use PDgrowth®:
If you miss a dose of PDgrowth®, skip that dose and take your next dose at the next prescribed time. Do not take an extra dose or increase the amount of your next dose to make up for the one you missed.
– Inject PDgrowth® right after reconstitution.
– Effect on Ability to Drive and Operate Machinery
Somatropin-containing products have no influence on the ability to drive and use machines.
– Overdosage
If you use too much PDgrowth® you may need medical treatment right away. Too much PDgrowth® can cause initial hypoglycemia, followed by hyperglycemia.
– If you forget to use PDgrowth®:
If you miss a dose of PDgrowth®, skip that dose and take your next dose at the next prescribed time. Do not take an extra dose or increase the amount of your next dose to make up for the one you missed.
– Inject PDgrowth® right after reconstitution.
The following recommendations are based on the applied dose in controlled clinical trials that demonstrated the efficacy of the treatment.
– GH deficiency in pediatric patients: by sub-cutaneous or intramuscular injection, 0.069–0.117 IU/kg daily or 0.7–1 mg/m2 daily.
– Turner syndrome in pediatric patients: by subcutaneous injection, 0.135–0.15 IU/kg daily or 1.4 mg/m2 daily.
– Prader-Willi syndrome in pediatric patients: by subcutaneous injection in children with growth velocity greater than 1 cm/year, in combination with energy-restricted diet, 0.105 IU/kg daily or 1 mg/m2 daily; max. 2.7 mg daily.
– Small pediatric patients regarding their gestational age: by subcutaneous injection, 0.105 IU/kg daily or 1 mg/m2 daily.
– Growth hormone deficiency in adult patients: by subcutaneous injection, initially 0.45–0.9 IU daily gradually increased if required to max. 3 IU daily; use minimum effective dose (requirements may decrease with age).
– Chronic renal insufficiency in pediatric patients: by subcutaneous injection, 0.135–0.15 IU/kg daily or 1.4 mg/m2 daily (higher doses may be needed) adjusted if necessary after 6 months.
– Wasting syndrome in AIDS patients: 0.26-0.3 IU/Kg per day or 0.3 IU/kg given as a daily subcutaneous injection.
– Idiopathic short stature: by subcutaneous injection 1.11-1.41 IU/kg divided into equal doses 6-7 days per week.
- PDgrowth® should not be administrated when there is evidence of active neoplastic. If there are neoplasia antecedents, PDgrowth® treatment should be initiated once the corresponding anti-neoplasia treatment is over.
- Sensibility to the drug and other ingredients
- Diabetic patients
- Patients who show acute respiratory failure
- Children patients with closed epiphysis
- If there is evidence of active intracranial tumor in past 12 months
- It is recommendable not to initiate PDgrowth® treatment either in patients suffering complications from open-cast method, abdominal surgery or traumatism
- In severely obese PWS patients or in those who show an important respiratory deterioration as confirmed through spirometry
- Active proliferative or severe nonproliferative diabetic retinopathy
PDgrowth® treatment should be indicated by a specialist physician who, based on appropriate tests, should verify the diagnosed growth hormone deficiency before treatment onset.
Under PDgrowth® therapy, a through control should be performed in these conditions:
- Patients with diabetes, family history of diabetes or obese patients
- Patients with glucose intolerance
- Some patients may develop hypothyroidism under PDgrowth® treatment; thus patients should undergo regular controls regarding thyroid function.
- Pediatric patients with endocrine disorders, including GHD, show a high incidence of luxation on femoral epiphysis. Under PDgrowth® treatments, presence of limp or pain development on hips or knees should be careful evaluated.
- Development of skin lesions, suspicious of malignity, should be monitored.
- Patients with history of scoliosis should be monitored for illness progression risk.
It is not known if PDgrowth® may harm your unborn child. If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine, as PDgrowth® should not be used during pregnancy. It is not known if the medicine in PDgrowth® passes into your milk. PDgrowth® should not be used if breast-feeding.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects. Adverse effects have been reported in less than 1% of the patients treated with the human recombinant growth hormone, mainly as transitorily reactions over the injection site.
- Acromegalic features on the face, hands and feet
- Diabetes
- Arthrosclerosis
- Arterial hypertension
- Carpal tunnel syndrome
- The development of antibodies
- Otitis media or hearing conditions in patients with Turner syndrome
- Gynecomastia
- Headache
- Articular and muscular pain
- Peripheral Edema
- Pancreatitis
- Rash
- Jaundice
- Intracranial hypertension
- Lypodystrophy on injection site
- Hematuria
- Hypothyroidism
- The concomitant administration of glucocorticoids may inhibit PDgrowth® effect growth. In case of associated ACTH production deficit, the treatment should be adjusted by means of glucocorticoids in order to minimize the inhibitory effect over growth.
- In diabetic patients, may cause resistance to insulin
- There is preliminary evidence of regulation of several cytochrome P 450 isoforms by the growth hormone. Potentially speaking, the growth hormone may alter the metabolism of some drugs which are metabolized by cytochrome p 450 systems. (Sexual hormones, Cyclosporin and Etc.)
- Elevated glycosolated Hgb (14%); increased AST (13%); increased fasting serum insulin levels (10%); increased levels of alkaline phosphatase, inorganic phosphate, and parathyroid hormone.
- Larger doses of somatropin may be required in women receiving oral estrogens.
Growth curve, Tanner staging (children), periodic thyroid function tests, bone age (annually), periodical urine testing for glucose, somatomedin C (IGF-I) levels; funduscopic examinations at initiation of therapy and periodically during treatment; serum phosphorus, alkaline phosphatase and parathyroid hormone. If growth deceleration is observed in children treated for growth hormone deficiency, and not due to other causes, evaluate for presence of antibody formation. Periodic blood glucose monitoring; strict blood glucose monitoring in patients with diabetes. Progression or recurrence of pre-existing tumors or malignant transformation of skin lesions.
CRI: Progression of renal osteodystrophy
Prader-Willi syndrome: Monitor for sleep apnea, respiratory infections, snoring (onset of or increased)
Turner syndrome: Ear disorders including otitis media; cardiovascular disorders
Noonan syndrome: Prior to use, verify short stature syndrome.
Mimics actions of naturally occurring growth hormone (GH) to stimulate linear and skeletal growth; increases number and size of skeletal muscle cells; increases RBC mass and internal organ size; increases cellular protein synthesis; reduces body fat stores and lipid mobilization, and increases plasma fatty acids. Somatropin, as well as the endogenous GH, functions by means of binding to specific receptors on the surface of numerous cells. The activation of these receptors triggers a cascade of intracellular events, particularly phosphorilations, which conclude on the regulation of the expression of several genes, at transcription level. The majority of somatropin anabolic effects are mediated by IGF-I, synthesized in the liver, and other tissues in response to the stimulation of GH receptors on its membrane, IGF-I concentrations are low in GHD pediatric patients, but they get to normal levels post somatropin treatment.
– Absorption : 70-90% in both injection route
– Half-life absorption : 2-4 hours ( depends on injection route )
– Distribution : about 1 L/Kg in adult patients
– Excretion: mainly excreted by renal and hepatic proteolysis. Approximately 0.1% of the dose excreted unaltered.
Reconstitute the content of a vial of PDgrowth® with one ml of water for injection from the ampoule with the solvent, making it flow against the vial wall. After reconstitution, swirls the vial with a soft rotary motion until the content is completely dissolved. Do not agitate the solution during preparation. Before administration, the stopper should be cleaned with a swab embedded in alcohol in order to avoid contamination. The injection should be given subcutaneously and the site varied to prevent lipoatrophy.
- Store in a refrigerator (2°C – 8°C)
- Keep the container in the outer carton in order to protect from light
- Keep out of the reach and sight of children.
- Do not use PDgrowth® after the expiry date which is stated on the box and on the syringe label (EXP). The expiry date refers to the last day of that month.
- Do not freeze PDgrowth® and do not use PDgrowth® that has been frozen or improperly refrigerated.
- Do not shake PDgrowth®
- Do not use PDgrowth® if you notice particles in the solution, or if it is cloudy or colored.
- PDgrowth® should inject right after reconstitution.
– Each ampoule with lyophilized PDgrowth® contains:
Somatropin – Glycine – Anhydrous sodium dibasic phosphate – Anhydrous sodium monobasic phosphate
– Each ampoule with diluent contains:
Distilled water for injection
Mechanism of action
Erythropoietin (EPO) is a glycoprotein hormone produced primarily by the kidney in response to hypoxia and is the key regulator of red blood cell (RBC) production. EPO is involved in all phases of erythroid development, and has its principal effect at the level of erythroid precursors. After EPO binds to its cell surface receptor, it activates signal transduction pathways that interfere with apoptosis and stimulates erythroid cell proliferation.
Pharmacokinetics
Absorption: About 20% in SC route. After subcutaneous administration, Cmax was achieved within 5 to 24 hours.
Distribution: About 9 L
Elimination: In adult and pediatric patients with CKD, the elimination half-life (t1/2) of plasma erythropoietin after intravenous administration of PDpoetin® ranged from 4 to 13 hours.
In Anemic cancer patients the elimination half-life (t1/2) of plasma erythropoietin after intravenous administration of PDpoetin® ranged from 16-67 hours.
The pharmacokinetic profile of PDpoetin® in children and adolescents appeared similar to that of adults.
The pharmacokinetics of PDpoetin® has not been studied in patients with HIV infection.
Guidance for Injection
PDpoetin® can be injected into your body using two different ways (routes) as described below. Follow your healthcare provider’s instructions about how you should inject PDpoetin®. In patients on hemodialysis, the intravenous (IV) route is recommended.
Before use, leave the PDpoetin® syringe to stand until it reaches room temperature. This usually takes between 15 and 30 minutes. Do not remove the syringe’s needle cover while allowing it to reach room temperature.
Only take one dose of PDpoetin® from each syringe.
PDpoetin® is given alone and not mixed with other liquids for injection.
Do not shake PDpoetin® Prolonged vigorous shaking may damage the product. If the product has been shaken vigorously, don’t use it.
Check the syringe, to make sure it is the right dose, has not passed its expiry date, is not damaged, and the liquid is clear and not frozen.
Choose an injection site. Do not inject PDpoetin® into an area that is tender, red, bruised, hard, or has scars or stretch marks. Recommended sites for injection are:
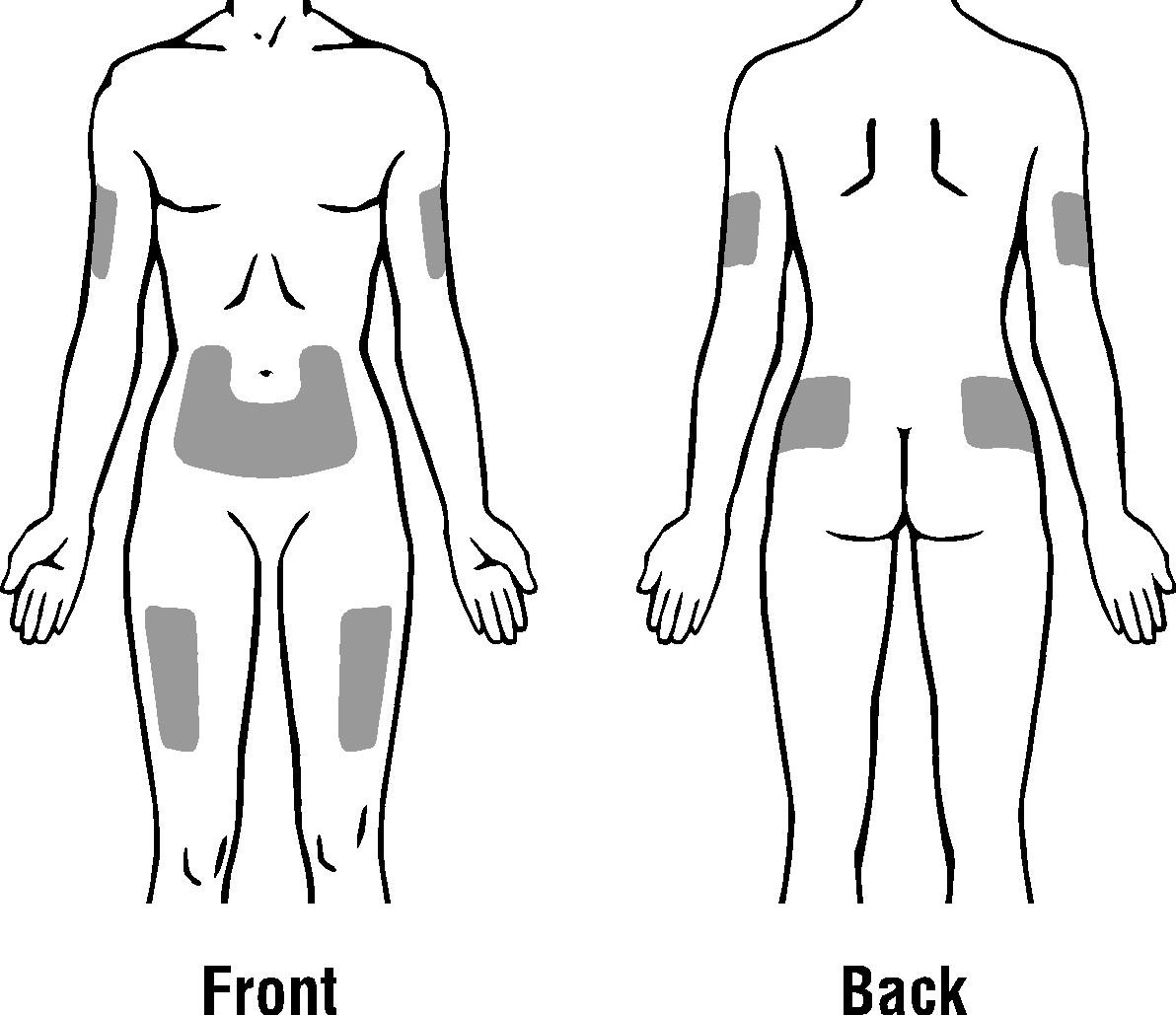
The outer area of the upper arms
The abdomen (except for the 2-inch area around the navel)
The front of the middle thighs
The upper outer area of the buttocks
Subcutaneous Route:
- Wash your hands. Use an antiseptic swab on the injection site, to disinfect it.
- Hold the pre-filled syringe by the body of the syringe with the covered needle pointing upward.
- Do not hold by the plunger head, plunger or needle cover.
- Do not pull back on the plunger at any time.
- Do not remove the needle cover from the pre-filled syringe until you are ready to inject your PDpoetin®.
- Take the cover off the syringe by holding the barrel and pulling the cover off carefully without twisting it. Don’t push the plunger, touch the needle or shake the syringe.
- Pinch a fold of skin between your thumb and index finger. Don’t squeeze it.
- Push the needle in fully. Your doctor or nurse may have shown you how to do this.
- Push the plunger with your thumb as far as it will go to inject the entire amount of liquid. Push it slowly and evenly, keeping the skin fold pinched.
- When the plunger is pushed as far as it will go, take out the needle and let go of the skin.
- When the needle is pulled out of your skin, there may be a little bleeding at the injection site. This is normal. You can press an antiseptic swab over the injection site for a few seconds after the injection.
- Dispose of your used syringe in a safe container.
Intravenous Route:
PDpoetin® can be injected in your vein through a special access port placed by your healthcare provider. This type of PDpoetin® injection is called an intravenous (IV) injection. This route is usually for hemodialysis patients.
- Wipe off the venous port of the hemodialysis tubing with an alcohol wipe.
- Insert the needle of the syringe into the cleaned venous port and pushes the plunger all the way down to inject all the PDpoetin®.
- Remove the syringe from the venous port. Do not recap the needle.
- Dispose of the used syringe and needle.
- Administer over at least one to five minutes, depending on the total dose.
- In hemodialysed patients, a bolus injection may be given during the dialysis session through a suitable venous port in the dialysis line. Alternatively, the injection can be given at the end of the dialysis session via the fistula needle tubing, followed by 10 ml of isotonic saline to rinse the tubing and ensure satisfactory injection of the product into the circulation.
- A slower administration is preferable in patients who react to the treatment with “flu-like” symptoms.
- Do not administer PDpoetin® by intravenous infusion or in conjunction with other drug solutions.
Storage and Stability
- Store in a refrigerator (2°C – 8°C).
- Keep the container in the outer carton in order to protect from light.
- Keep out of the reach and sight of children.
- Do not use PDpoetin® after the expiry date which is stated on the box and on the syringe label (EXP). The expiry date refers to the last day of that month.
- Do not freeze PDpoetin® and do not use PDpoetin® that has been frozen or improperly refrigerated.
- Do not shake PDpoetin®.
- PDpoetin® pre-filled syringes are single dose containers, discard any unused product.
Other Composition
Human Albumin – Sodium Chloride – Sodium Citrate